
A more modern method of compressing hydrogen, which doesn’t require mechanical force, is electrochemical compression. Electrochemical compression involves using a proton exchange membrane (pem), surrounded by electrodes, to pull low-pressure hydrogen through the membrane into a highly pressurized container. Research has demonstrated that using electrochemical compression is cheaper and more effective than mechanical compression and it is expected to become a key
hydrogen compression
technology in the future. Presently, electrochemical hydrogen compressors are capable of compressing hydrogen to pressures as high as 5,000 psi. It is believed that once the technique has been fully developed pressures beyond 10,000 psi will be attainable.
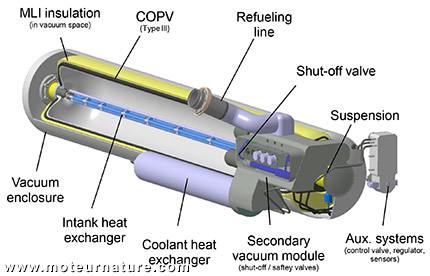
Hydrogen gas supply pressures are often lower than the use pressure whether supplied by tube trailers, pipeline, or a liquid hydrogen tank. Compressors are used to increase pressure of hydrogen gas. Compressors are commonly driven by electric motors, pneumatic cylinders, or hydraulic cylinders. When electric motor drive systems are used, they must meet the electric classification requirements for equipment in a hydrogen environment. Electrochemical hydrogen compressors represent an emerging technology that may be suitable for some future applications. Considerations for compressor installation include: combustible compressor lubricants can present a fire hazard. The compressor discharged pressure should be monitored by a control system and shut down the compressor when the discharge pressure reaches the target value.
Both hydrogen compression and storage are currently major topics for the implementation of a hydrogen economy. Hydrogen carriers as mhs, lohcs, ammonia or cryo-adsorption in acs and mofs may play an important role for the development of safe, reliable and compact alternatives to current technologies for hydrogen compression and storage. While conventional mechanical compressors are a mature solution, they still have several limitations. The presence of moving parts leads to increased maintenance costs, as well as higher noise and vibration. Current alternatives include ionic liquid, mh and electrochemical compressors, as well as combinations of these. The use of ionic liquids with almost no solubility for hydrogen is a very smart solution to avoid gas leakage.
Hydrogen and Fuel Cell Technologies Office Home
Much of chemistry can be thought of as a molecular game of musical chairs. When atoms get together to form new molecules, their electrons rearrange themselves to make and break the bonds between them. Scientists rely on computers and intricate calculations to simulate those interactions. In this virtual lab, they can explore different molecular configurations before investing time and resources in experiments. At johnson matthey, an early azure quantum elements customer, scientists use those simulations to discover and develop sustainable technologies like hydrogen fuel cells and catalysts that can slash emissions from cars and trucks. “if you think about the materials discovery process, you can go into the lab and tinker with different chemicals to try and make something, or you can use fundamental physics to screen the entire periodic table,” said misbah sarwar, research lead for johnson matthey’s physical and chemical modeling team.

Storing electrical energy as hydrogen fuel is now feasible at high density with hyet’s compression technology. Hydrogen energy storage is the solution for a multitude of intermittent or distributed, renewable energy sources to address the seasonal character of renewables + grid flexibility. Even a small amount of surplus energy can be captured and stored with greater efficiency than at rated capacity.
Unlike fossil fuels, which are extracted as an energy-producing natural resource, hydrogen must be synthesized. This is because it occurs naturally in low atmospheric concentrations and capturing hydrogen is not economically viable for the scale required in the energy sector. Instead, it is produced by breaking down other molecules like methane or water using industrial processes. Therefore, hydrogen cannot serve as a net source of renewable energy—its only utility is in its ability to store energy. Hydrogen stores 33. 6 megawatt hours (mwh) of energy per ton of hydrogen. However, the current best available technology to produce hydrogen electrolytically requires approximately 48 mwh per ton of hydrogen.